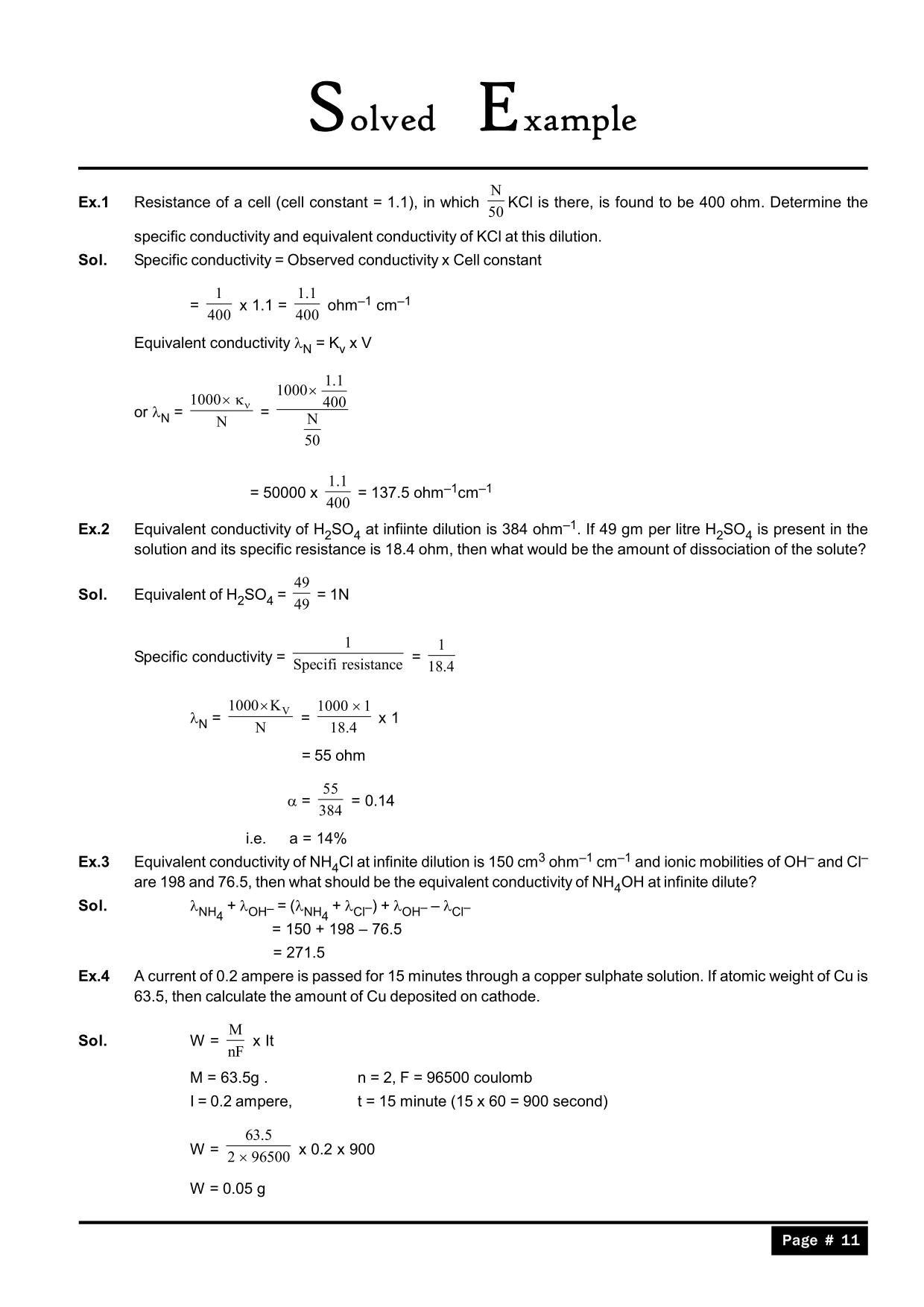
Electrochemistry Practice Test Grade 12 . In the ionic compound ammonium dichromate, (nh4)2cr2o7? Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. Study with quizlet and memorize flashcards containing terms like what are the rules for oxidation numbers?, balance: Terms in this set (67) oxidation. Electrochemical reactions july 2022 question paper topic test. The loss of electrons by a chemical entity. Free printable redox reactions and electrochemistry worksheets for 12th grade. At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. The gain of electrons by a chemical enitity. What is the oxidation number of chromium.
from www.esaral.com
What is the oxidation number of chromium. Electrochemical reactions july 2022 question paper topic test. Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. Study with quizlet and memorize flashcards containing terms like what are the rules for oxidation numbers?, balance: In the ionic compound ammonium dichromate, (nh4)2cr2o7? The loss of electrons by a chemical entity. Terms in this set (67) oxidation. The gain of electrons by a chemical enitity. At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. Free printable redox reactions and electrochemistry worksheets for 12th grade.
Electrochemistry Notes for Class 12, IIT JEE & NEET
Electrochemistry Practice Test Grade 12 Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. Electrochemical reactions july 2022 question paper topic test. In the ionic compound ammonium dichromate, (nh4)2cr2o7? What is the oxidation number of chromium. Terms in this set (67) oxidation. The gain of electrons by a chemical enitity. Study with quizlet and memorize flashcards containing terms like what are the rules for oxidation numbers?, balance: Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. The loss of electrons by a chemical entity. At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. Free printable redox reactions and electrochemistry worksheets for 12th grade.
From www.youtube.com
electrochemical cell electrochemistry class 12 chemistry subject notes Electrochemistry Practice Test Grade 12 What is the oxidation number of chromium. Electrochemical reactions july 2022 question paper topic test. Free printable redox reactions and electrochemistry worksheets for 12th grade. The loss of electrons by a chemical entity. Terms in this set (67) oxidation. In the ionic compound ammonium dichromate, (nh4)2cr2o7? Study with quizlet and memorize flashcards containing terms like what are the rules for. Electrochemistry Practice Test Grade 12.
From byjus.com
Class 12 Chemistry Worksheet on Chapter 3 Electrochemistry Set 3 Electrochemistry Practice Test Grade 12 In the ionic compound ammonium dichromate, (nh4)2cr2o7? At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. The loss of electrons by a chemical entity. Study with quizlet and memorize flashcards containing terms like what are the rules for oxidation numbers?, balance: Discover the world of redox reactions and electrochemistry. Electrochemistry Practice Test Grade 12.
From www.esaral.com
Electrochemistry Notes for Class 12, IIT JEE & NEET Electrochemistry Practice Test Grade 12 Terms in this set (67) oxidation. Electrochemical reactions july 2022 question paper topic test. At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. Free printable redox reactions and electrochemistry worksheets for 12th grade. Study with quizlet and memorize flashcards containing terms like what are the rules for oxidation numbers?,. Electrochemistry Practice Test Grade 12.
From www.youtube.com
Electrochemistry grade 12 multiple choice questions YouTube Electrochemistry Practice Test Grade 12 The gain of electrons by a chemical enitity. The loss of electrons by a chemical entity. In the ionic compound ammonium dichromate, (nh4)2cr2o7? Electrochemical reactions july 2022 question paper topic test. What is the oxidation number of chromium. At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. Free printable. Electrochemistry Practice Test Grade 12.
From www.studypool.com
SOLUTION Class 12th electrochemistry practice worksheet Studypool Electrochemistry Practice Test Grade 12 Free printable redox reactions and electrochemistry worksheets for 12th grade. Electrochemical reactions july 2022 question paper topic test. The loss of electrons by a chemical entity. Study with quizlet and memorize flashcards containing terms like what are the rules for oxidation numbers?, balance: Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. In the ionic. Electrochemistry Practice Test Grade 12.
From byjus.com
NCERT Exemplar Class 12 NCERT Exemplar Chemistry Solutions Chapter 3 Electrochemistry Practice Test Grade 12 The gain of electrons by a chemical enitity. Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. Study with quizlet and memorize flashcards containing terms like what are the rules for oxidation numbers?, balance: At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. Free. Electrochemistry Practice Test Grade 12.
From byjus.com
Class 12 Chemistry Worksheet on Chapter 3 Electrochemistry Set 4 Electrochemistry Practice Test Grade 12 Free printable redox reactions and electrochemistry worksheets for 12th grade. Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. In the ionic compound ammonium dichromate, (nh4)2cr2o7? The gain of electrons by a chemical enitity. What is the oxidation number of chromium. Terms in this set (67) oxidation. The loss of electrons by a chemical entity.. Electrochemistry Practice Test Grade 12.
From byjus.com
NCERT Exemplar Class 12 NCERT Exemplar Chemistry Solutions Chapter 3 Electrochemistry Practice Test Grade 12 Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. Free printable redox reactions and electrochemistry worksheets for 12th grade. The loss of electrons by a chemical entity. At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. Terms in this set (67) oxidation. Study with. Electrochemistry Practice Test Grade 12.
From studylib.net
Electrochemistry Practice Sheet VIJETA SERIES CLASS12TH Electrochemistry Practice Test Grade 12 The gain of electrons by a chemical enitity. In the ionic compound ammonium dichromate, (nh4)2cr2o7? At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. The loss of electrons by a chemical entity. Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. Free printable redox. Electrochemistry Practice Test Grade 12.
From quizizz.com
50+ redox reactions and electrochemistry worksheets for 12th Grade on Electrochemistry Practice Test Grade 12 Electrochemical reactions july 2022 question paper topic test. Study with quizlet and memorize flashcards containing terms like what are the rules for oxidation numbers?, balance: Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. The loss of electrons by a chemical entity. At standard conditions, ecell = e°cell because all the concentrations are 1 m. Electrochemistry Practice Test Grade 12.
From www.jagranjosh.com
Class 12 Chemistry Electrochemistry NCERT Exemplar Solutions Electrochemistry Practice Test Grade 12 Electrochemical reactions july 2022 question paper topic test. The gain of electrons by a chemical enitity. What is the oxidation number of chromium. Terms in this set (67) oxidation. At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. In the ionic compound ammonium dichromate, (nh4)2cr2o7? Study with quizlet and. Electrochemistry Practice Test Grade 12.
From vidyakul.com
Class 12th Chemistry Electrochemistry NCERT Notes CBSE 2023 Electrochemistry Practice Test Grade 12 Study with quizlet and memorize flashcards containing terms like what are the rules for oxidation numbers?, balance: Free printable redox reactions and electrochemistry worksheets for 12th grade. In the ionic compound ammonium dichromate, (nh4)2cr2o7? The gain of electrons by a chemical enitity. What is the oxidation number of chromium. Terms in this set (67) oxidation. At standard conditions, ecell =. Electrochemistry Practice Test Grade 12.
From www.jagranjosh.com
CBSE 12th Chemistry Board Exam 2020 Important Questions & Answers from Electrochemistry Practice Test Grade 12 In the ionic compound ammonium dichromate, (nh4)2cr2o7? Study with quizlet and memorize flashcards containing terms like what are the rules for oxidation numbers?, balance: Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. Terms in this set (67) oxidation. The loss of electrons by a chemical entity. What is the oxidation number of chromium. The. Electrochemistry Practice Test Grade 12.
From www.youtube.com
Electrochemistry grade 12 Exam Questions YouTube Electrochemistry Practice Test Grade 12 Electrochemical reactions july 2022 question paper topic test. Free printable redox reactions and electrochemistry worksheets for 12th grade. In the ionic compound ammonium dichromate, (nh4)2cr2o7? Terms in this set (67) oxidation. The gain of electrons by a chemical enitity. At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. What. Electrochemistry Practice Test Grade 12.
From www.scribd.com
12TH GRADE ELECTROCHEMISTRY WORKSHEET1 (2) PDF Electrochemistry Electrochemistry Practice Test Grade 12 Terms in this set (67) oxidation. The gain of electrons by a chemical enitity. What is the oxidation number of chromium. Electrochemical reactions july 2022 question paper topic test. Discover the world of redox reactions and electrochemistry with our free printable worksheets, tailored. The loss of electrons by a chemical entity. At standard conditions, ecell = e°cell because all the. Electrochemistry Practice Test Grade 12.
From studylib.net
Electrochemistry Practice Test Key Electrochemistry Practice Test Grade 12 The gain of electrons by a chemical enitity. Terms in this set (67) oxidation. Free printable redox reactions and electrochemistry worksheets for 12th grade. In the ionic compound ammonium dichromate, (nh4)2cr2o7? What is the oxidation number of chromium. At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. Discover the. Electrochemistry Practice Test Grade 12.
From www.lessonplanet.com
Electrochemistry Handout for 9th 12th Grade Lesson Electrochemistry Practice Test Grade 12 What is the oxidation number of chromium. In the ionic compound ammonium dichromate, (nh4)2cr2o7? Free printable redox reactions and electrochemistry worksheets for 12th grade. The gain of electrons by a chemical enitity. At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. Study with quizlet and memorize flashcards containing terms. Electrochemistry Practice Test Grade 12.
From www.esaral.com
Electrochemistry Class 12 Important Questions with Answers Electrochemistry Practice Test Grade 12 What is the oxidation number of chromium. At standard conditions, ecell = e°cell because all the concentrations are 1 m at an ambient temperature of 273 k. In the ionic compound ammonium dichromate, (nh4)2cr2o7? Electrochemical reactions july 2022 question paper topic test. Study with quizlet and memorize flashcards containing terms like what are the rules for oxidation numbers?, balance: Discover. Electrochemistry Practice Test Grade 12.